Like catching two fish with one worm, treating two problems with a single drug is efficient, but exceedingly difficult. In particular, for new diabetes medications, in which one drug aims to tackle two major complications of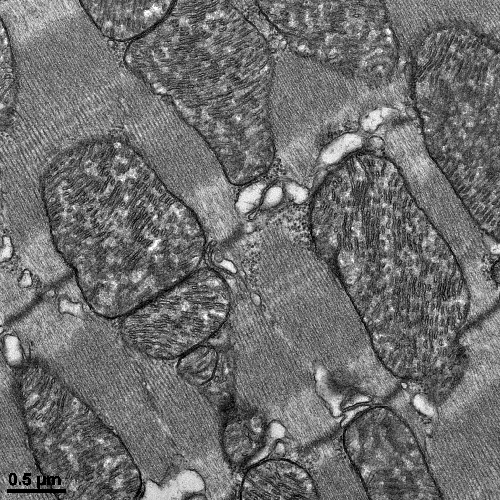 diabetes – the excess of both lipids and glucose in the blood – the therapeutic benefits, while great, frequently are accompanied by dangerous toxic effects to the heart.
diabetes – the excess of both lipids and glucose in the blood – the therapeutic benefits, while great, frequently are accompanied by dangerous toxic effects to the heart.
Why and how these drugs, known as dual PPARα/γ agonists cause heart dysfunction in diabetes patients has been unclear. But now, in new research published in the journal JCI Insight, scientists at the Lewis Katz School of Medicine at Temple University (LKSOM) show for the first time that dual PPARα/γ diabetes drugs have a profound toxic effect on the generation and function of mitochondria, the tiny energy factories that power cells.
“We found that the combined activation of PPARα and PPARγ receptors by a single agonist drug, tesaglitazar, blocked the activity of proteins involved in mitochondrial biogenesis and energy production, including a protein known as SIRT1,” explained Konstantinos Drosatos, PhD, Assistant Professor of Pharmacology and Assistant Professor in the Center for Translational Medicine and the Center for Metabolic Disease Research at LKSOM and senior investigator on the new study. “When we reactivated SIRT1 with resveratrol, an antioxidant widely known for its presence in grape skins, heart toxicity was reduced and the benefits of dual lowering of lipid and glucose levels were maintained in tesaglitazar-treated mice.”
The effects of PPARα and PPARγ receptor activation are like the fish that researchers are trying to bait. The PPARα receptor binds molecules such as fibrates, which help reduce blood triglyceride levels and increase levels of high-density lipoproteins (HDLs) – popularly known as “heart-healthy” fats. Meanwhile, PPARγ receptors attach molecules that help lower blood glucose levels.
The popular diabetes drugs known as thiazolidinediones (TZDs), which include pioglitazone and rosiglitazone (the latter marketed as Avandia), bind to PPARγ receptors. Because these drugs given alone have been questioned for cardiac toxicity, the idea emerged for dual PPARα/γ activation by a single drug – the one piece of bait that in theory successfully lures the two fish – the combined lipid- and glucose-lowering effects of PPARα/γ coactivation.
To understand why these new drugs are accompanied by just as much cardiac toxicity, if not more, than TZDs, Dr. Drosatos and colleagues carried out a series of studies in diabetic mice treated with the dual PPARα/γ agonist tesaglitazar. Despite reduced triglyceride and glucose levels in the blood, the mice developed cardiac dysfunction. Molecular analyses of heart tissue from affected animals revealed a significant reduction in the expression and activation of a protein known as cardiac PPARγ coactivator 1-α (PGC1α), which plays a critical role in mitochondrial biogenesis. This reduction was accompanied by decreases in SIRT1 expression and in mitochondrial abundance.
The researchers then repeated their experiment, this time treating diabetic mice with tesaglitazar in combination with resveratrol, which serves as an activator of SIRT1. Mice treated with the combination of the two drugs had reduced heart toxicity, relative to tesaglitazar-only therapy, and their heart cells exhibited normal mitochondrial function.
“Now we have much a clearer idea of how heart toxicity arises from treatment with dual PPARα/γ agonists,” Dr. Drosatos said. “This allows us to more effectively guide the development of future PPAR-targeting drugs.”
One of the team's next aims is to further elucidate the signaling pathway that moderates the effects of PPAR drugs in order to identify a single target – one worm to catch one really big fish. “By targeting fewer proteins, there should be fewer toxic effects to worry about,” Dr. Drosatos added.
Other researchers who contributed to the study include Charikleia Kalliora, Center for Translational Medicine, LKSOM, and Faculty of Medicine, University of Crete; Ioannis D. Kyriazis, Melissa J. Lieu, Yujia Yue, Christine J. Pol, Ying Tian, and Muniswamy Madesh, Center for Translational Medicine, Department of Pharmacology, LKSOM; Shin-ichi Oka, Wataru Mizushima, Adave Chin, and Junichi Sadoshima, Cardiovascular Research Institute, Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School; Estela Area-Gomez, Department of Neurology, Columbia University Irving Medical Center, New York; Diego Scerbo, Division of Preventive Medicine and Nutrition, Columbia University, and the NYU Langone School of Medicine, Division of Endocrinology, Diabetes and Metabolism; Ira J. Goldberg, NYU Langone School of Medicine, Division of Endocrinology, Diabetes and Metabolism; P. Christian Schulze, Department of Internal Medicine I, Division of Cardiology, Angiology, Intensive Medical Care and Pneumology, University Hospital Jena, Germany; and Mete Civelek, Center for Public Health Genomics, Department of Biomedical Engineering, University of Virginia.
The research was supported in part by National Heart, Lung, and Blood Institute/National Institutes of Health grants HL112853, HL130218, HL073029, and HL135987 and by a grant from the W.W. Smith Charitable Trust.
Image description: electron microscopy image showing that combined activation of PPARα and PPARγ by the antidiabetic drug tesaglitazar causes cardiac dysfunction by compromising function of mitochondria.
