The human genetic blueprint is like a string of code. To follow it, the code, or DNA, is transcribed into shorter strings of RNA. While some of these shorter strings carry instructions for making proteins – the functional units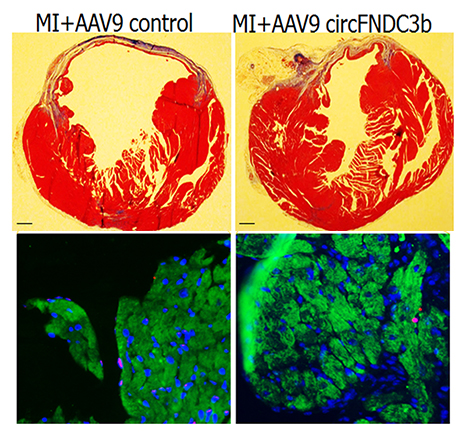 of cells – most RNA is not involved in protein production. Among these noncoding RNAs are the recently discovered circular RNAs, so-named because of their unusual ring shape (most other RNAs are linear).
of cells – most RNA is not involved in protein production. Among these noncoding RNAs are the recently discovered circular RNAs, so-named because of their unusual ring shape (most other RNAs are linear).
Circular RNAs, like other noncoding RNAs, were thought to be nonfunctional, but recent evidence suggests otherwise. Circular RNAs may in fact act like sponges to “soak up,” or bind, other molecules, including microRNAs and proteins, and now, new work by researchers at the Lewis Katz School of Medicine at Temple University (LKSOM) and colleagues supports this idea. They describe, for the first time, a circular RNA that fills a critical role in tissue repair after heart attack, thanks to its ability to soak up harmful molecules.
The study was published online September 20 in the journal Nature Communications.
“We discovered that a circular RNA known as circFndc3b, when added therapeutically to the injured heart after surgically induced heart attack in mice, enhances cardiac repair and helps restore heart function,” explained Raj Kishore, PhD, Professor of Pharmacology and Medicine and Director of the Stem Cell Therapy Program in the Center for Translational Medicine at LKSOM and senior investigator on the new report. “We attributed these effects of circFndc3b to its ability to function like a 'sponge,' binding a protein called FUS that mediates cell death and reduces vascular growth, which hinders heart tissue repair.”
Dr. Kishore and colleagues focused their investigation on circFndc3b after finding that this particular circular RNA was significantly decreased in the heart in mice that had experienced a heart attack. “This observation led us to wonder whether the change in circFndc3b expression meant that it was important functionally in the heart,” Dr. Kishore said.
To investigate this possibility, a gene product to induce circFndc3b overexpression was injected into the heart in mice after heart attack. Subsequent examination showed that within eight weeks of injection, treated mice experienced gains in heart function and in survival compared to their untreated counterparts. There was also evidence within heart tissue that new blood vessels had started to form, greatly aiding the tissue repair process.
The findings offer exciting insight into circular RNAs and the significance of their potential role as molecular sponges that limit the activity of damaging molecules. “CircFndc3b specifically soaked up an RNA binding protein that suppresses blood vessel formation,” Dr. Kishore explained. “In doing so, it made way for new vessels to grow.”
Dr. Kishore and colleagues are now in the process of developing a large animal model to further investigate the therapeutic potential of circFndc3b. The team also wants to begin analyzing plasma samples from patients just after heart attack to investigate whether specific circulating RNAs could serve as biomarkers for heart disease or injury and to get a better sense of their clinical significance.
Other researchers who contributed to the study include Venkata Naga Srikanth Garikipati, Zhongjian Cheng, May M. Truongcao, Maria Cimini, Yujia Yue, Grace Huang, Chunlin Wang, Cindy Benedict, Yan Tang, Vandana Mallaredy, Jessica Ibetti, Erhe Gao, and Sudarsan Rajan, Center for Translational Medicine, LKSOM; Steven Houser, Cardiovascular Research Center and Department of Physiology, LKSOM; Walter J. Koch, the Center for Translational Medicine and Department of Pharmacology, LKSOM; Suresh Kumar Verma, Division of Cardiovascular Diseases, Department of Medicine, The University of Alabama at Birmingham; Dongming Liang and Jeremy E. Wilusz, Department of Biochemistry and Biophysics, University of Pennsylvania Perelman School of Medicine; Laurel Grisanti, Department of Biomedical Sciences, University of Missouri; Sarah M. Schumacher, Lerner Research Institute, Cleveland Clinic; and David Goukassian, Zena & Michael A. Weiner Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York.
The research was supported in part by National Institutes of Health grants HL091983, HL126186, and HL134608.
Image description: injection of circFNDC3b in mouse hearts after myocardial infarction reduces the area of infarct (top panel, right) and reduces the cardiac muscle cell death (bottom panel, dead muscle indicated by magenta color).
